 For spherical nanoparticles, the volume is: V=4/3r 3, where r is the radius of the sphere. Standard temperature and pressure conditions defined by NIST are to be used, as specified in the problem statement. Molar mass of KMnO 4 = 39.1 g + 54.9 g Now the liters of solution is needed. The molar mass (M) is a physical property and it is defined as the mass of one mole of the chemical substance or it is a ratio of the mass of a chemical compound to its amount of chemical substance. For example: Make a 50 mL solution of 0.75 molar NaCl. How to Use the Molar Mass Calculator?
For spherical nanoparticles, the volume is: V=4/3r 3, where r is the radius of the sphere. Standard temperature and pressure conditions defined by NIST are to be used, as specified in the problem statement. Molar mass of KMnO 4 = 39.1 g + 54.9 g Now the liters of solution is needed. The molar mass (M) is a physical property and it is defined as the mass of one mole of the chemical substance or it is a ratio of the mass of a chemical compound to its amount of chemical substance. For example: Make a 50 mL solution of 0.75 molar NaCl. How to Use the Molar Mass Calculator? The procedure to use the molar mass calculator is as follows: M -molar mass Calculated molar mass for the selected ratio of propane and butane in the LPG mixture Calculation setup Select value to calculate. Conditions: The piston of a single-stage, single-cylinder and single-action compressor has diameter d = 200 mm and stroke s = 150 mm. The volume of 100 g of Sodium hydroxide solution: 100 1. In related terms, another unit of mass often used is Dalton (Da) or unified atomic mass unit (u) when describing atomic masses and molecular masses. A classical case is ethanol + water.
 Example problem: Molar mass of K = 39.1 g; Molar mass of Mn = 54.9 g; Molar mass of O = 16.0 g (The solute contains 4 O atoms, so count the 16g 4 times.) As far as I recall (thermodynamics) the volume of the mixture is based on partial molar volumes and it may not be equal to the sum of the volumes of the components.
Example problem: Molar mass of K = 39.1 g; Molar mass of Mn = 54.9 g; Molar mass of O = 16.0 g (The solute contains 4 O atoms, so count the 16g 4 times.) As far as I recall (thermodynamics) the volume of the mixture is based on partial molar volumes and it may not be equal to the sum of the volumes of the components. 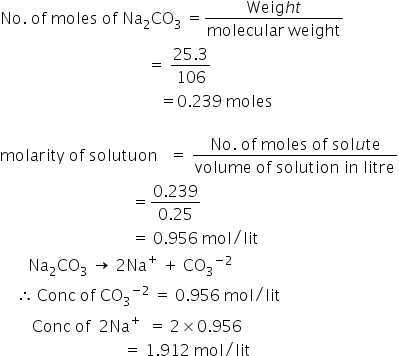 P combustion heat power calculation q liq/q gas/ LPG flow rate calculation Select value to input. Subtract the volume of the stock solution from the desired final volume.
P combustion heat power calculation q liq/q gas/ LPG flow rate calculation Select value to input. Subtract the volume of the stock solution from the desired final volume.  Step 1: Calculate the volume of 100 grams of Sodium Hydroxide solution. To figure this out, you will need the molar mass of NaCl which is 58.44 g/mol. Rated this article: Share yours! You should enter selected one. "Clarified the calculation for me."
Step 1: Calculate the volume of 100 grams of Sodium Hydroxide solution. To figure this out, you will need the molar mass of NaCl which is 58.44 g/mol. Rated this article: Share yours! You should enter selected one. "Clarified the calculation for me." Air is a mixture of several gases, where the two most dominant components in dry air are 21 vol% oxygen and 78 vol% nitrogen.Oxygen has a molar mass of 15.9994 g/mol A calculation of quantity of heat transferred can rely on a hypothetical quantity of energy transferred as adiabatic work and on the first law of thermodynamics. Compressor shaft rotates at n = 120 rpm. Molar mass of CaCl 2, m 2 = 111 g mol-1. Calculation procedure On completion of measurement calculation of the total cargo quantity can be carried out.
Volume = Weight Density.
As mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. In thermodynamics, the specific heat capacity (symbol c p) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity.Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The molecular weight (or molar mass) of a substance is the mass of one mole of the substance, and can be calculated by summarizing the molar masses of all the atoms in the molecule.. The mass formula is given as Mass = v. Where, = density and. Molar volume: 22.392 dm/mol. The percent by weight formula aids in the calculation of the relative percent concentration of the solute dissolved in the solution. For normal samples from earth with typical isotope composition, the atomic weight can be approximated by the standard atomic weight or the conventional atomic weight.. M(H) = 1.007 And, for a given temperature and pressure, the molar volume is the same for all ideal gases, and is known to the same precision as the gas constant: R = 0.082 057 338(47) L atm K1 mol1, that is a relative standard uncertainty of 5.7107, according to the 2014 C is the molar concentration in mol/L (Molar or M). Make sure that you count the atoms for each element and calculate the molar mass of each of the atoms. Here is how to perform the calculation. The molar volume of an ideal gas at standard temperature and pressure (273.15 K, 101.325 kPa) is 22.413 962 x 10-3 m3 mol-1 with a standard uncertainty of 0.000013 x 10-3 m3 mol-1 2. The molar mass of atoms of an element is given by the relative atomic mass of the element multiplied by the molar mass constant, M u = 0.999 999 999 65 (30) 10 3 kgmol 1. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. To calculate this molar volume, Eq. For rod shaped nanoparticles, the volume is:V=r 2 l, where r is the radius of the rod and l is the length. Wurtzite [ZnS or SZn] weighs 3 980 kg/m (248.46328 lb/ft) [ weight to volume | volume to weight | price | mole to volume and weight | mass and molar concentration | density] Volume to weight, weight to volume and cost conversions for Refrigerant R-414B, liquid (R414B) with temperature in the range of -40C (-40F) to 71.12C (160.016F) Wurtzite [ZnS or SZn] weighs 3 980 kg/m (248.46328 lb/ft) [ weight to volume | volume to weight | price | mole to volume and weight | mass and molar concentration | density] Volume to weight, weight to volume and cost conversions for Refrigerant R-414B, liquid (R414B) with temperature in the range of -40C (-40F) to 71.12C (160.016F) CAS Registry Number (CAS RN): 7782-44-7. Example, at ambient conditions: Henry's law is a gas law formulated by the British chemist William Henry in 1803. A 1 M solution is one in which exactly 1 mole of solute is dissolved in a total solution volume The mole, symbol mol, is the SI base unit of amount of substance. Problem 2: Sodium Hydroxide. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M. You can also use this molarity calculator to find the mass concentration or molar mass. v = the volume. The calculation of MMSCFD, first requires the standard temperature and pressure conditions to be fixed. The calculator below uses the formula to convert liters to moles You can calculate the volume you need to add if you acid is 100 percent pure by relationship density is equal to mass divided by volume. Calculation of dead volume in a piston compressor. Find the molar mass of each element using the periodic table of elements. Acquisto on-line da un'ampia selezione presso il negozio CD e Vinili. One mole of sodium (Na) is 22.99 g, and 1 mole of chlorine is 35.45 g. For sodium chloride (NaCl) they are in a ratio of 1:1 so the molar mass of NaCl is BYJUS online molar volume calculator tool performs the calculation faster, and it displays the molar volume in a fraction of seconds. If you know that titrating 50.00 ml of an HCl solution requires 25.00 ml of 1.00 M NaOH, you can calculate the concentration of hydrochloric acid, HCl. The molar mass of a compound is simply the mass of the number of molecules of the compound. Formula: Density = W e i g h t V o l u m e. OR. Keep in mind, this is the total volume of the solution, not the volume of solvent used to dissolve the solute. For example, a pH of about 4.0 with a buffer strength of 10 mM is obtained using 0.12% anhydrous citric acid and 0.11% trisodium citrate dihydrate. Percent weight by volume; The percent weight by volume gives us the weight per volume ratio. It is also abbreviated as %w/v. BYJUS online molar mass calculator tool makes the calculation faster, and it displays the molar mass in a fraction of seconds. There is no internationally agreed standard for gas cargo calculations and procedures can vary particularly with the chemical gases. The calculation uses the ideal gas equation: The ideal gas equation is a good approximation for many common gases. The molar mass of isopropyl alcohol and water is 60 g mol 1 and 18 g mol 1. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron.For example, if beaker A contains 10 An isopropyl alcohol solution contains 40 g of isopropyl alcohol and 20 g of water. The simple calculation starts from 0.73 cm 3 /g x 10 24 A/cm 3 x molecular weight g/mole-----6.02 x 10 23 molecules/mole and results in a protein volume of approximately: (1.21 x MW) A 3 /molecule This provides a reasonable estimate for general uses. You should enter not selected one. Learn about what a molarity is and how to calculate the molarity of a solution with an example calculation. Since 2019, a mole of any substance is the amount of that substance containing an exactly defined number of particles, N = 6.022140761023. The law states that at a constant temperature, the amount of dissolved gas in a volume of a specified liquid is directly proportional to the partial pressure of the gas in equilibrium with the liquid. The air inside is compressed at pressure from P1 = 0.1 MPa to P2 = 0.32 MPa. The exponents m and n are How to find molar mass. Understand the Beer-Lambert law for absorbance, A = x l x c. The standard equation for absorbance is A = x l x c, where A is the amount of light absorbed by the sample for a given wavelength, is the molar absorptivity, l is the distance that the light travels through the solution, and c is the concentration of the absorbing species per unit volume. What is molar mass? It is defined to be 1/12 of the mass of one atom of carbon-12 and in older works is also abbreviated as "amu". Press the calculate button, and the approximate percentages of anhydrous citric acid and trisodium citrate dihydrate will be displayed in %weight/volume. Copy and paste this code into your website. For plate shaped nanoparticles, the volume is V=r 2 h , where r is the radius of the nanoplate and h You can see from the equation there is a 1:1 molar ratio between HCl and NaOH. Standard Temperature T S = 0 Molar Mass Calculator is a free online tool that displays the molar mass of the chemical compound. The density of the mixture is, of course, equal to the total mass divided by the volume of the mixture. The molar volume of an ideal gas at 1 atmosphere of pressure is 0.022 413 969 545 014 m 3 /mol at 0 C, 0.024 465 403 697 038 (the calculation is performed automatically by the structure determination software).
The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The molarity of a sodium hydroxide solution is 0.51 M. The molar mass of sodium hydroxide is 40 g mol 1. You will use the final volume of the solution to calculate the number of grams needed to make your molar solution. Note: 50% (w/w) Sodium Hydroxide means that 100 g of solution contains 50 g of Sodium Hydroxide. How to Use the Molar Volume Calculator? First, convert the grams to moles using the molar mass and then use Avogadro's number to find the number of molecules: This calculation tells you that there are 2.1 x 10 22 molecules of NaCl in 2 The unit of molar mass is kg/mol.
Components in Dry Air.
M HCl x volume HCl = M NaOH x volume NaOH. In other words, the amount of dissolved gas is directly proportional to the partial pressure of its gas phase. 515 = 66.0066 ml. Here k(T) is the reaction rate constant that depends on temperature, and [A] and [B] are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. The molar mass is the mass in grams of 1 mole of a particular molecule. In this chapter, we will learn more about molar mass formula & calculation of molar mass Such calculation is the primary approach of many theoretical studies of quantity of heat transferred. Calculation of the Enthalpy Change on Mixing where v is the molar volume of the mixture at T, P, and z. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) Bookmarks: [ weight to volume | volume to weight | price | mole to volume and weight | mass and molar concentration | density ] A few materials, substances, compounds or elements with a name containing, like or similar to Oxygen: The standard molar enthalpy of formation of a compound is defined as the enthalpy of formation of 1.0 2.3.4.2.